It’s less than a week away from Floyd Mayweather, Jr. vs Manny Pacquiao, and the world is trembling in anticipation of the Fight to Watch for this generation! Personally, the adrenaline rush I get when I view all those promo videos (official and fan made ones) is mind blowing. Talk about butterflies in the stomach early in the morning in the absence of any threat! However, it is this very feeling that will be the subject of this post at the end of April 2015. How often are we subject to ‘threatening’ situations where our basic instinct of survival kicks in? Not so much? Well, even during sports, especially when we are put on the spot, where winning or losing a major game would fall entirely on our shoulders… that’s when Adrenaline can make or break us. Would we succumb to panic? Or would the hormone steer our heightened senses to victory?
 An interesting infographic that depicts the stages of the Fight or Flight Response. The symptoms seem rather debilitating and detrimental to our survival. However, if they are honed and focused, they can bring us back to what we may have been created originally to be, not the domesticated creatures we are now.
An interesting infographic that depicts the stages of the Fight or Flight Response. The symptoms seem rather debilitating and detrimental to our survival. However, if they are honed and focused, they can bring us back to what we may have been created originally to be, not the domesticated creatures we are now.
Now, firstly we need to address the actual system itself. Our Autonomic Nervous System is a control system that acts largely unconsciously and regulates heart rate, digestion, respiratory rate, pupillary response, urination, and sexual arousal. The role of this system is mediated by the Sympathetic Nervous System, which has the main function of activating the physiological changes that occur during the fight or flight response, and the Parasympathetic Nervous System, which has the main function of activating the “rest and digest” response and return the body to homeostasis after the fight or flight response. Basically like an “ON” (norepinephrine) and “OFF” (acetylcholine) switch respectively.

Norepinephrine

Acetylcholine
Now imagine the build up to the fight on May 2nd. Neither of them will run. They’ve been wanting this for years! Once they are both in the ring, let’s take a look at what will happen.
The amygdala will trigger a neural response in the hypothalamus. The initial reaction is followed by activation of the pituitary gland and secretion of the hormone adrenocorticotropic hormone (ACTH). The adrenal gland is activated almost simultaneously and releases the neurotransmitter epinephrine. The release of chemical messengers results in the production of the hormone cortisol, which increases blood pressure, blood sugar, and suppresses the immune system (AKA ENERGY BOOST!). Epinephrine basically binds with liver cells and glucose production spikes. Additionally, the circulation of cortisol functions to turn fatty acids into available energy, which prepares muscles throughout the body for response. Catecholamine hormones, such as adrenaline (epinephrine) or noradrenaline (norepinephrine), facilitate immediate physical reactions associated with a preparation for violent muscular action.
There is a huge list of these, which includes acceleration of heart and lung action, dilation of blood vessels for muscles, pupil dilation, tunnel vision (this can be a problem, which requires focus and training to hone), loss of hearing (better known as auditory exclusion as you focus only on the sounds you need!), shaking (well DUH!), inhibition of erection (not THIS is interesting!). Also, you will have increased strength and speed! Awesome, isn’t it?
However, as with all things, nothing is free. During the reaction, the intensity of emotion brought about by the stimulus may be too high for most. Individuals with high levels of emotional reactivity may be prone to anxiety and aggression. A burst of energy such as these ones would require some form of trade off. It leaves us feeling drained after. Tired. Weak. Wet pants (for some).
Do you have problems with butterflies in the stomach? Anxiety? Stress? Unable to stand up in public or unable to solve your big problems due to fear? Then you have lost to adrenaline… rather than befriended and used it. Fear is our greatest enemy, but we were all born with the tools to combat it. Use your fear, channel it into something positive. Transcend your past self, and FIGHT!












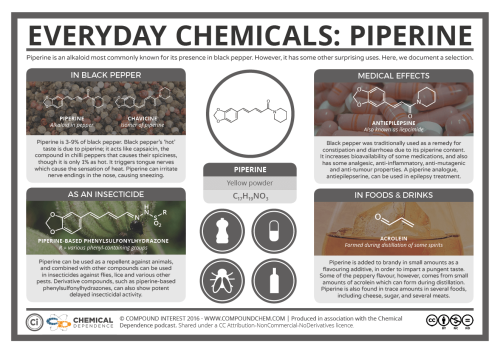
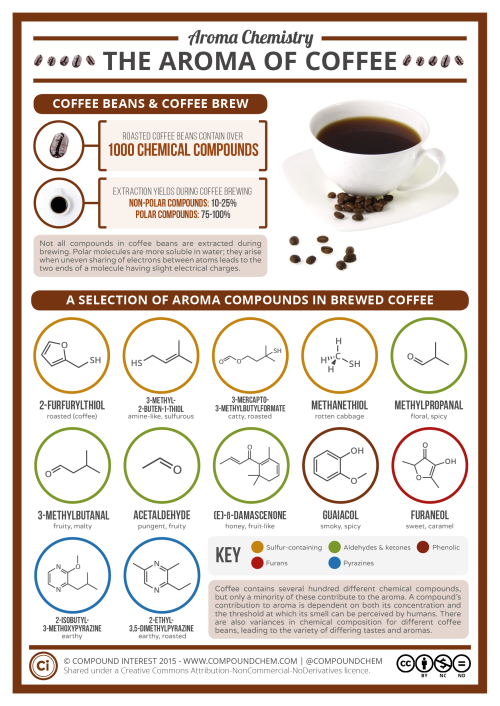
 Now to start off, I just simply HAVE to mention caffeine, the world’s most widely consumed psychoactive drug. More specifically, it is a central nervous stimulant (CNS) of the methylxanthine class of psychoactive drugs. As a substance, it is a bitter, white crystalline purine, a methylxanthine alkaloid and closely related to adenine, which is found in DNA and RNA. What’s important is that it can stave off drowsiness, definitely useful in many situations. But it also has negative side effects like insomnia, gastric, etc. Nothing is free in this world… what we have to do is weigh the pluses and minuses… and decide!
Now to start off, I just simply HAVE to mention caffeine, the world’s most widely consumed psychoactive drug. More specifically, it is a central nervous stimulant (CNS) of the methylxanthine class of psychoactive drugs. As a substance, it is a bitter, white crystalline purine, a methylxanthine alkaloid and closely related to adenine, which is found in DNA and RNA. What’s important is that it can stave off drowsiness, definitely useful in many situations. But it also has negative side effects like insomnia, gastric, etc. Nothing is free in this world… what we have to do is weigh the pluses and minuses… and decide!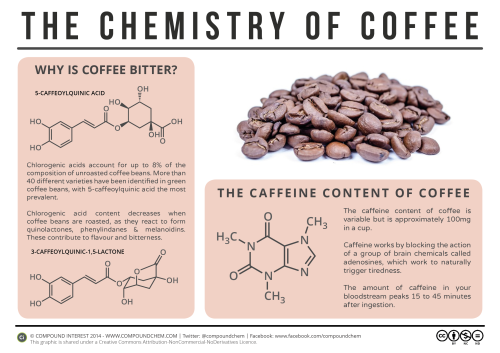

 An interesting infographic that depicts the stages of the Fight or Flight Response. The symptoms seem rather debilitating and detrimental to our survival. However, if they are honed and focused, they can bring us back to what we may have been created originally to be, not the domesticated creatures we are now.
An interesting infographic that depicts the stages of the Fight or Flight Response. The symptoms seem rather debilitating and detrimental to our survival. However, if they are honed and focused, they can bring us back to what we may have been created originally to be, not the domesticated creatures we are now.